
Chemistry, 26.10.2021 14:00 angeladominguezgarci
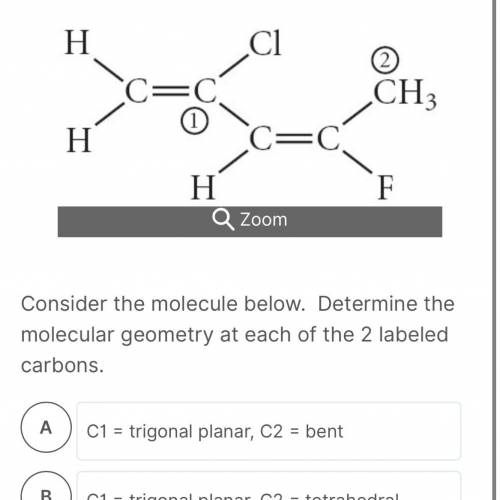
Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. HELP ME PLEASE ASAP
C1 = trigonal planar, C2 = bent
B
C1 = trigonal planar, C2 = tetrahedral
C
C1 = bent, C2 = trigonal planar
D
C1 = tetrahedral, C2 = linear

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. HELP...
Questions

History, 14.04.2020 05:46

English, 14.04.2020 05:48

Mathematics, 14.04.2020 05:48



Mathematics, 14.04.2020 05:48

Mathematics, 14.04.2020 05:48


Mathematics, 14.04.2020 05:48

Chemistry, 14.04.2020 05:48


History, 14.04.2020 05:48

Mathematics, 14.04.2020 05:48


Mathematics, 14.04.2020 05:48

Chemistry, 14.04.2020 05:48




Mathematics, 14.04.2020 05:49



