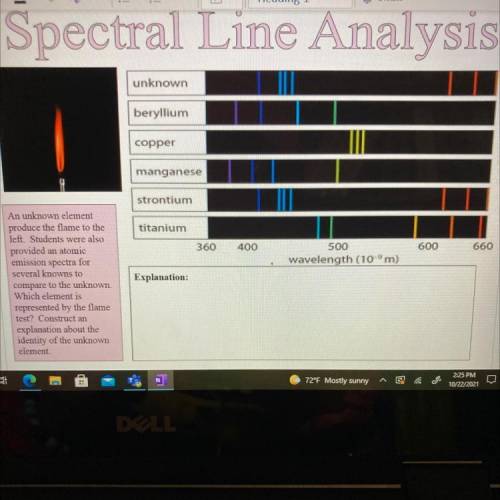
An unknown element
produce the flame to the
left. Students were also
provided an atomi...

Chemistry, 24.10.2021 08:20 jennelledenise
An unknown element
produce the flame to the
left. Students were also
provided an atomic
emission spectra for
several knowns to
compare to the unknown.
Which element is
represented by the flame
test? Construct an
explanation about the
identity of the unknown
element.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1



Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
Questions


Mathematics, 09.04.2020 15:15




History, 09.04.2020 15:15

Biology, 09.04.2020 15:15

Social Studies, 09.04.2020 15:15

English, 09.04.2020 15:15


Medicine, 09.04.2020 15:15

Mathematics, 09.04.2020 15:16





Mathematics, 09.04.2020 15:16

Mathematics, 09.04.2020 15:16




