
Chemistry, 24.10.2021 05:10 sfcsullivan9466
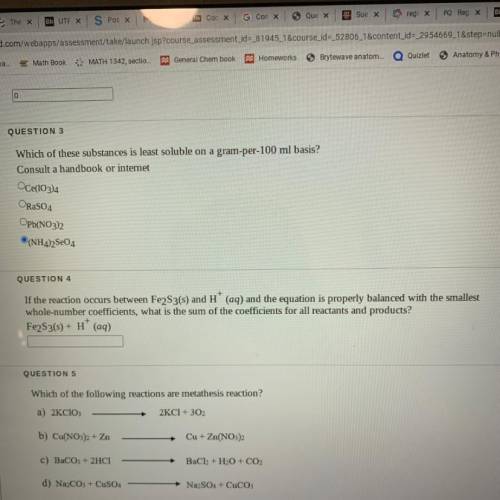
If the reaction occurs between Fe2S3(s) and H+ (aq) and the equation is properly balanced with the smallest
whole-number coefficients, what is the sum of the coefficients for all reactants and products?
Fe2S3(s) + H+ (aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

You know the right answer?
If the reaction occurs between Fe2S3(s) and H+ (aq) and the equation is properly balanced with the s...
Questions

Mathematics, 19.03.2020 04:34





English, 19.03.2020 04:34





Mathematics, 19.03.2020 04:35


Mathematics, 19.03.2020 04:35


Mathematics, 19.03.2020 04:35





Advanced Placement (AP), 19.03.2020 04:36



