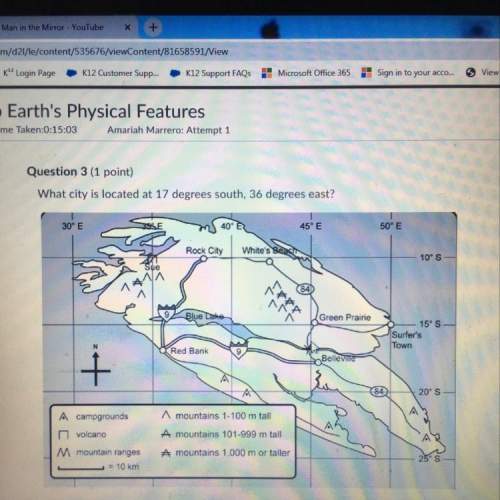
Chemistry, 20.10.2021 09:40 kitykay2399
Copper reacts with concentrated nitric acid.
Cu(s) + + 4HNO3(aq) + Cu(NO3)2(aq) + 2NO2(9) + 2H2O(1)
An excess of copper is added to 25.0 cm3 of 16.0 mol/dm3 HNO3.
Use this information, together with the equation above, to calculate the volume of NO, formed.
The gas volume is measured at room temperature and pressure.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Copper reacts with concentrated nitric acid.
Cu(s) + + 4HNO3(aq) + Cu(NO3)2(aq) + 2NO2(9) + 2H2O(1...
Questions


Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

History, 21.11.2020 01:00









Mathematics, 21.11.2020 01:00


Advanced Placement (AP), 21.11.2020 01:00

Health, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Arts, 21.11.2020 01:00