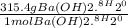WILL GIVE BRAINLIEST AND 20 POINTS! Barium hydroxide, often used to titrate weak organic acids, is obtained as the octahydrate, Ba(OH)2 * 8 H2O. What mass of Ba(OH)2 * 8 H2O would be required to make 500 mL of a solution that is 0.1500 M hydroxide ions? [hint: calculate the molar mass of barium hydroxide octahydrate].

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
WILL GIVE BRAINLIEST AND 20 POINTS!
Barium hydroxide, often used to titrate weak organic acids, is...
Questions

Mathematics, 05.03.2021 18:30

Health, 05.03.2021 18:30



Mathematics, 05.03.2021 18:30




Mathematics, 05.03.2021 18:30

Mathematics, 05.03.2021 18:30

Mathematics, 05.03.2021 18:30

History, 05.03.2021 18:30

Mathematics, 05.03.2021 18:30

Mathematics, 05.03.2021 18:30

History, 05.03.2021 18:30

English, 05.03.2021 18:30

Mathematics, 05.03.2021 18:30

Mathematics, 05.03.2021 18:30


 ×
× ×
×