
Chemistry, 18.10.2021 08:30 untouchedyannaa
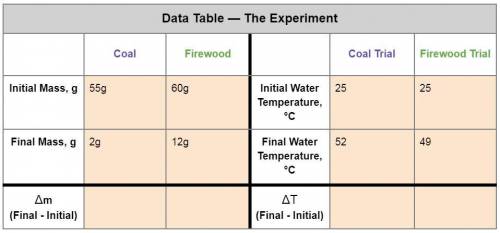
1. Calculate the heat gained by the water when the Firewood was burned.
Equation: q=mc(T f-Ti)
q = heat (J)
m = mass of the water = 50g
c is a constant = 4.184
T f = final temperature
Ti = initial temperature
-
2. Calculate the heat gained by the water when the Coal was burned.
Equation: q=mc(T f-Ti)
q = heat (J)
m = mass of the water = 50g
c is a constant = 4.184
T f = final temperature
Ti = initial temperature

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1
You know the right answer?
1. Calculate the heat gained by the water when the Firewood was burned.
Equation: q=mc(T f-Ti)
Questions

Mathematics, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

History, 20.09.2020 16:01



Social Studies, 20.09.2020 16:01

History, 20.09.2020 16:01

History, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01



Mathematics, 20.09.2020 16:01


Computers and Technology, 20.09.2020 16:01

English, 20.09.2020 16:01

Geography, 20.09.2020 16:01

History, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01



