
Chemistry, 17.10.2021 08:10 nicolecoulthard
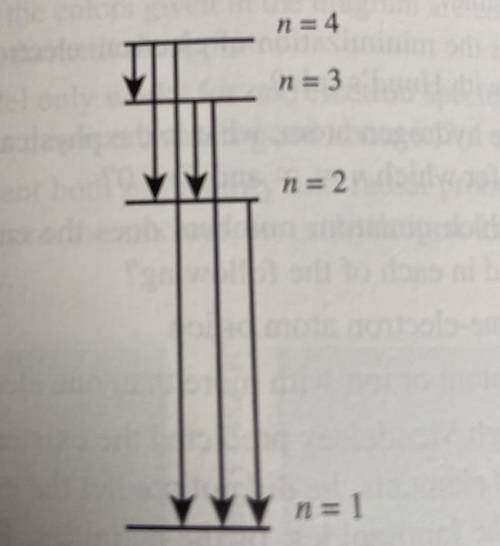
Consider only the transitions involving the first four energy levels for a hydrogen atom:
a) How many emissions are possible for an electron in the n=4 level as it goes to the ground state
b)which electronic transition is the lowest energy?
c) Which electronic transition corresponds to the shortest wavelength emission

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Consider only the transitions involving the first four energy levels for a hydrogen atom:
a) How m...
Questions


Mathematics, 10.03.2020 21:25

Mathematics, 10.03.2020 21:25


Social Studies, 10.03.2020 21:25

Mathematics, 10.03.2020 21:25


Physics, 10.03.2020 21:25









Business, 10.03.2020 21:26





