
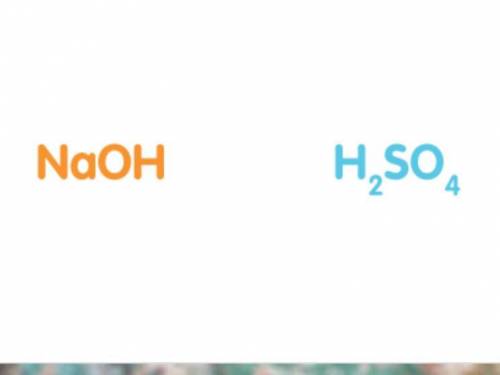
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentration of the sodium hydroxide solution was 20g/dm3. Work out the concentration of the acid in moles per litre if it took 25cm3 of acid to completely neutralise the alkali. (Hint: It may help to write out a balanced symbol equation for the reaction. The chemical formulas of the acid and alkali are shown below.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 14:00
Comparing john newland’s octaves with the modern periodic table, which 5 elements have been discovered between hydrogen and iron since newland’s time?
Answers: 3

Chemistry, 23.06.2019 14:20
Identificaa 5 características que comparten las guacamayas y dos caracteristicas que las hagan diferentes
Answers: 1
You know the right answer?
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentrati...
Questions

Engineering, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01




Mathematics, 22.10.2020 23:01

Chemistry, 22.10.2020 23:01

Health, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01



Physics, 22.10.2020 23:01

Chemistry, 22.10.2020 23:01

English, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01

English, 22.10.2020 23:01




