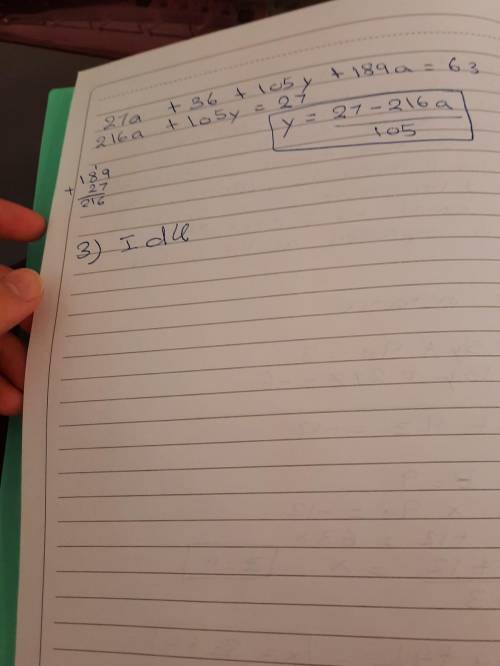1-Link the concepts of mole, molar mass, number of atoms, Avogadro’s
number and mass using a flowchart or mind map.
2-Rearrange the two equations to make each variable the focus (you will end up with three variations for each equation).
3- Record the equations for number of moles and number of particles (and
each equation triangle) - Annotate each symbol with what it means and definition and the units.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
1-Link the concepts of mole, molar mass, number of atoms, Avogadro’s
number and mass using a flowc...
Questions

Mathematics, 21.02.2020 23:34


Spanish, 21.02.2020 23:34








English, 21.02.2020 23:34

History, 21.02.2020 23:34