
Chemistry, 11.10.2021 18:40 donnafranks2003
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 13.19 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2


Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2....
Questions



Computers and Technology, 28.02.2021 17:10

Mathematics, 28.02.2021 17:10


Mathematics, 28.02.2021 17:10


Chemistry, 28.02.2021 17:10


Mathematics, 28.02.2021 17:10

Mathematics, 28.02.2021 17:10

Mathematics, 28.02.2021 17:10

English, 28.02.2021 17:10






Physics, 28.02.2021 17:10

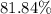 .
. .
.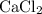 , as well as those in the product of interest,
, as well as those in the product of interest, 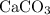 :
: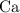 :
: 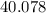 .
.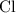 :
: 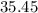 .
. :
: 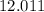 .
. :
:  .
.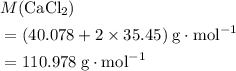 .
.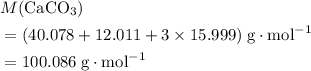 .
.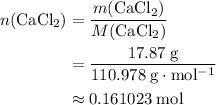 .
. ) are both
) are both  . Thus:
. Thus: .
. 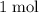 of
of 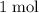 of
of 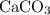 in this experiment:
in this experiment: .
. .
. , calculate the percentage yield of this experiment:
, calculate the percentage yield of this experiment: .
.

