Please help me with this!
Information needed to
A pharmaceutical spectroscopist place...

Chemistry, 11.10.2021 14:00 lizzepacheco
Please help me with this!
Information needed to
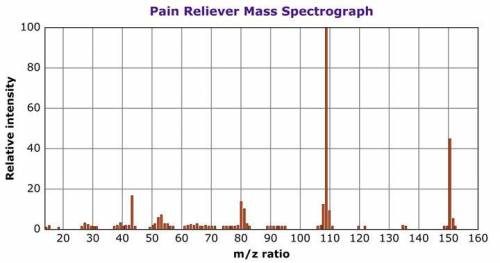
A pharmaceutical spectroscopist places a sample of a pain reliever into a mass spectrometer and obtains data about different compounds within the sample. The image below shows a mass spectrograph of the data. The relative intensity, shown on the vertical axis, indicates the relative amount of each compound in the sample. The m/z (mass to charge) ratio, shown on the horizontal axis, is related to the molecular mass of each compound.
Question:
Describe two observations you can make from the graph. (Graph is below)
As usual, any non-answers will be reported. Thank you!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Questions


Mathematics, 13.10.2019 16:50






Mathematics, 13.10.2019 16:50




English, 13.10.2019 16:50

Mathematics, 13.10.2019 16:50




Mathematics, 13.10.2019 16:50





