
Chemistry, 06.10.2021 22:20 tatertottheyoungin
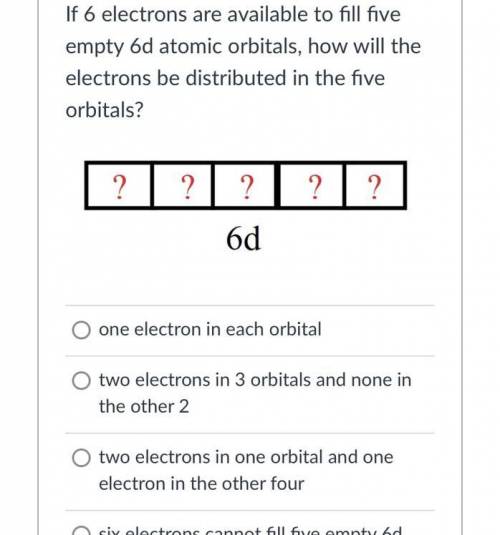
If 6 electrons are available to fill fiveempty 6d atomic orbitals, how will theelectrons be distributed in the fiveorbitals?? ? ? 2 ?6dO one electron in each orbitalO two electrons in 3 orbitals and none inthe other 2two electrons in one orbital and oneelectron in the other fourO six electrons cannot fill five empty 6datomic orbitals

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
If 6 electrons are available to fill fiveempty 6d atomic orbitals, how will theelectrons be distribu...
Questions


Mathematics, 08.03.2021 06:30

Mathematics, 08.03.2021 06:30

Mathematics, 08.03.2021 06:30

Mathematics, 08.03.2021 06:30

English, 08.03.2021 06:30



Mathematics, 08.03.2021 06:30



Biology, 08.03.2021 06:30

Biology, 08.03.2021 06:30

Mathematics, 08.03.2021 06:30

Health, 08.03.2021 06:30

Mathematics, 08.03.2021 06:30


Arts, 08.03.2021 06:30


Mathematics, 08.03.2021 06:30



