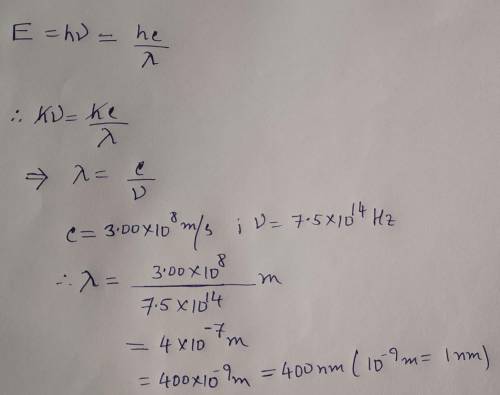Chemistry, 06.10.2021 09:50 juliabochkarev
A photon of blue light has a frequency of 7.50 × 10¹⁴ Hz. Calculate the wavelength of this photon (c = 3.00 × 10⁸ m/s) in nanometers. please show work

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

You know the right answer?
A photon of blue light has a frequency of 7.50 × 10¹⁴ Hz. Calculate the wavelength of this photon (c...
Questions

Biology, 05.02.2022 15:50




Mathematics, 05.02.2022 16:00

History, 05.02.2022 16:00

Advanced Placement (AP), 05.02.2022 16:00


Biology, 05.02.2022 16:00


Social Studies, 05.02.2022 16:00

Chemistry, 05.02.2022 16:00

Social Studies, 05.02.2022 16:00

Mathematics, 05.02.2022 16:00

Mathematics, 05.02.2022 16:00


Geography, 05.02.2022 16:00