
Chemistry, 04.10.2021 14:00 daniel1480
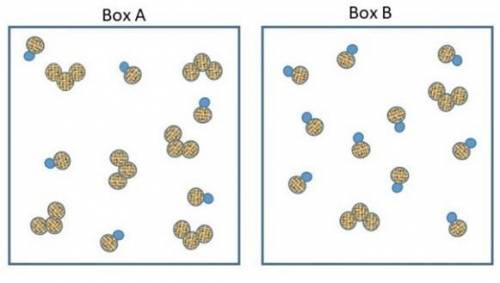
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g) → O2 (g) + NO2 (g) with a rate law: Rate = k (O3 )(NO)
Which flask will react faster than the other? Determine how much faster the fast one reacts compared to the slow one? Explain your answers in terms of molecular collisions.
How do I find the answer to this? My thinking is that box a will react more since there's equal amounts of reactants but box b will react faster since there's so much NO to collide with the O3 molecules. Thanks.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

You know the right answer?
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g...
Questions

Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00

Biology, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00


Health, 27.10.2020 01:00




Mathematics, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00





History, 27.10.2020 01:00





