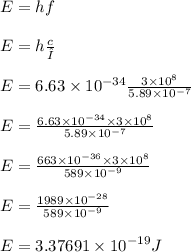Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.
...

Chemistry, 04.10.2021 08:40 Lilabsterdoll
Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
Questions


Mathematics, 01.08.2019 18:00



Mathematics, 01.08.2019 18:00



History, 01.08.2019 18:00



Mathematics, 01.08.2019 18:00

Social Studies, 01.08.2019 18:00

Mathematics, 01.08.2019 18:00

Mathematics, 01.08.2019 18:00


Mathematics, 01.08.2019 18:00


Arts, 01.08.2019 18:00