
Chemistry, 03.10.2021 01:30 nathangirnet
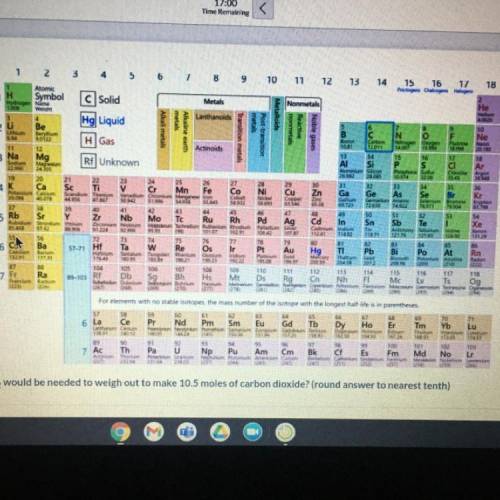
What mass of CO2 would be needed to weigh out to make 10.5 moles of carbon dioxide? (round answer to nearest tenth)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
What mass of CO2 would be needed to weigh out to make 10.5 moles of carbon dioxide? (round answer to...
Questions


Mathematics, 03.04.2021 03:00

Mathematics, 03.04.2021 03:00

Mathematics, 03.04.2021 03:00





Social Studies, 03.04.2021 03:00

Mathematics, 03.04.2021 03:00

History, 03.04.2021 03:00

Mathematics, 03.04.2021 03:00











