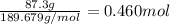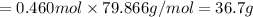Chemistry, 29.09.2021 21:20 Rflaig1129841
When TiCl4 (s) reacts with H20 (), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g of
titanium (IV) chloride present, with water in excess, how much solid titanium (IV) oxide (in grams)
could theoretically be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
When TiCl4 (s) reacts with H20 (), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g...
Questions



Health, 04.08.2019 07:30

Social Studies, 04.08.2019 07:30

Biology, 04.08.2019 07:30

Physics, 04.08.2019 07:30

Health, 04.08.2019 07:30

Chemistry, 04.08.2019 07:30



Social Studies, 04.08.2019 07:30

Mathematics, 04.08.2019 07:30

Mathematics, 04.08.2019 07:30


Mathematics, 04.08.2019 07:30

Biology, 04.08.2019 07:30



Biology, 04.08.2019 07:30

English, 04.08.2019 07:30