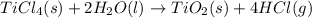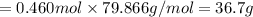Chemistry, 29.09.2021 21:10 kobiemajak
When TiCl4 (s) reacts with H20 (l), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g of
titanium (IV) chloride present, with water in excess, how much solid titanium (IV) oxide (in grams)
could theoretically be produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

You know the right answer?
When TiCl4 (s) reacts with H20 (l), the products TiO2 (s) and HCl (g) are formed. If there is 87.3 g...
Questions

Physics, 08.07.2019 14:30

Spanish, 08.07.2019 14:30

Mathematics, 08.07.2019 14:30



History, 08.07.2019 14:30



Health, 08.07.2019 14:30

English, 08.07.2019 14:30


English, 08.07.2019 14:30


Computers and Technology, 08.07.2019 14:30






Chemistry, 08.07.2019 14:40