1 point
3. According to Table 3, how do the different types of 5-carbon molecules
differ wit...

Chemistry, 28.09.2021 20:30 soonerlady19
1 point
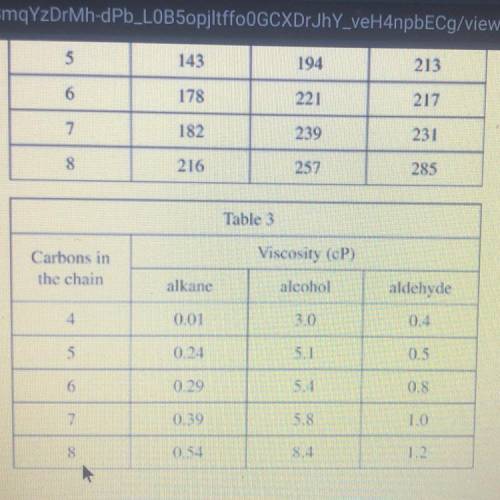
3. According to Table 3, how do the different types of 5-carbon molecules
differ with respect to their viscosity?
The alkane has a higher viscosity than the aldehyde and the aldehyde has a higher
viscosity than the alcohol.
The alkane has a higher viscosity than the alcohol and the alcohol has a higher
viscosity than the aldehyde
The alcohol has a higher viscosity than the alkane and the alkane has a higher
viscosity than the aldehyde.
The alcohol has a higher viscosity than the aldehyde and the aldehyde has a higher
viscosity than the alkane

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1
You know the right answer?
Questions


Computers and Technology, 18.12.2019 19:31







Social Studies, 18.12.2019 19:31

Computers and Technology, 18.12.2019 19:31








Computers and Technology, 18.12.2019 19:31


Computers and Technology, 18.12.2019 19:31



