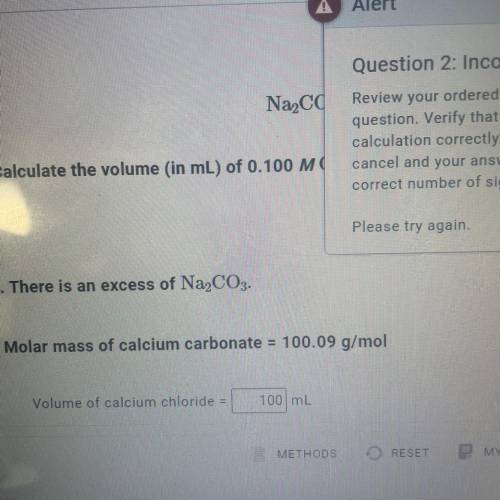
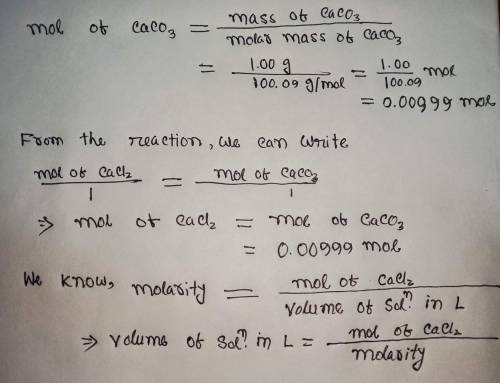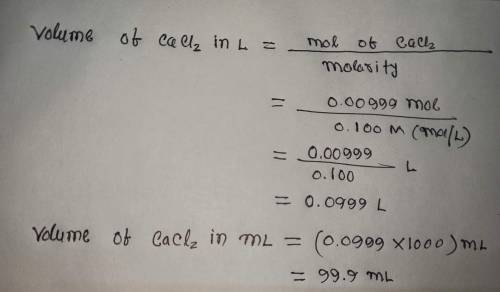Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There...

Chemistry, 28.09.2021 03:10 hammackkatelyn60
Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
*The answer is not 100ml or 10ml. Somehow the rounding up is not working well.*

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Questions


Mathematics, 27.01.2021 21:40


Chemistry, 27.01.2021 21:40

Mathematics, 27.01.2021 21:40

Mathematics, 27.01.2021 21:40

Mathematics, 27.01.2021 21:40

Mathematics, 27.01.2021 21:40





Mathematics, 27.01.2021 21:40

Mathematics, 27.01.2021 21:40





Mathematics, 27.01.2021 21:40

Business, 27.01.2021 21:40