
Chemistry, 27.09.2021 19:10 anjumuddin9
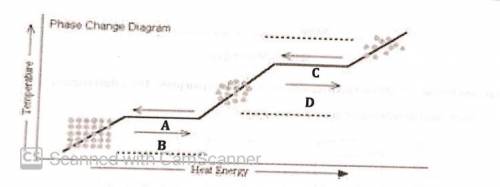
9. In the phase change diagram above, the horizontal lines represent
A. A constant temperature of a substance as it goes through a phase change.
B. A change in temperature of a substance as it goes through a phase change.
C. A period when no phase change occurs.
"A period when heat is not absorbed.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
9. In the phase change diagram above, the horizontal lines represent
A. A constant temperature of...
Questions

Mathematics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20


Mathematics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20



Engineering, 29.01.2021 03:20





Mathematics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20


Mathematics, 29.01.2021 03:20

Mathematics, 29.01.2021 03:20



