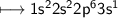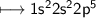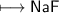6. Neon has 10 electrons - 2 in the inner shell and 8 in the outer shell. This arrangement of 8 electrons in the
outer shell is extremely stable and makes neon inert, with a valence of zero. Sodium has 11 electrons - 2
in the first shell, 8 in the next shell, and I in the outer shell. Fluorine has 9 electrons - 2 in the first shell
and 7 in the outer shell. How could sodium and fluorine from a compound in which both elements would
be like neon with 8 electrons in the outer shell?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
6. Neon has 10 electrons - 2 in the inner shell and 8 in the outer shell. This arrangement of 8 elec...
Questions

History, 29.10.2019 03:31





Geography, 29.10.2019 03:31

History, 29.10.2019 03:31

History, 29.10.2019 03:31

Mathematics, 29.10.2019 03:31






Computers and Technology, 29.10.2019 03:31



Biology, 29.10.2019 03:31

Chemistry, 29.10.2019 03:31