
Chemistry, 27.09.2021 14:00 xscape0mari
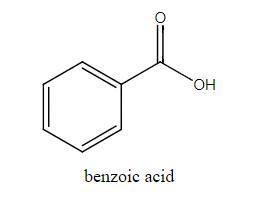
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in benzene is twice what we would expect for the molecular formula, C7H6O2. Explain this apparent anomaly. Thank you! :)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
The molar mass of benzoic acid (C6H5COOH) determined by measuring the freezing‐point depression in b...
Questions








Chemistry, 20.08.2020 01:01


Mathematics, 20.08.2020 01:01

History, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01







Mathematics, 20.08.2020 01:01



