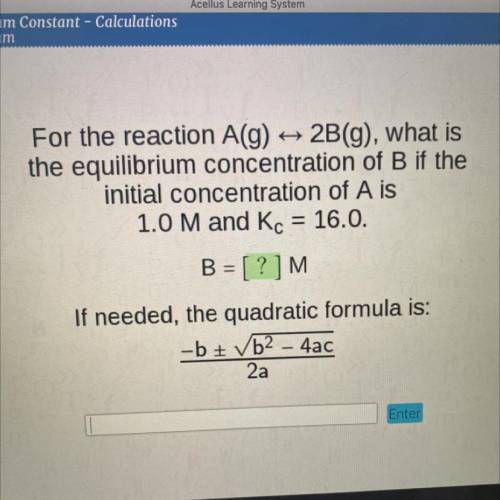
For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial...

For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial concentration of A is
1.0 M and Kc = 16.0.
B = [?]M
If needed, the quadratic formula is:
-b + b2 - 4ac
2a
PLEASE HELP IM SO CONFUSED AND NO LESSON VIDEOS ARE HELPFULL
ILL RATE YOU BRAINLIEST

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Questions












Social Studies, 27.07.2019 07:00





World Languages, 27.07.2019 07:00


Mathematics, 27.07.2019 07:00

Mathematics, 27.07.2019 07:00



