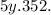Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
A student uses a variety of techniques to separate four different compounds from 2.10 g of a mixture...
Questions


Business, 22.04.2020 22:07



Physics, 22.04.2020 22:07

Mathematics, 22.04.2020 22:07


Social Studies, 22.04.2020 22:07

History, 22.04.2020 22:07


Mathematics, 22.04.2020 22:07




English, 22.04.2020 22:07





Mathematics, 22.04.2020 22:07