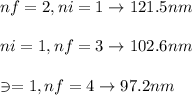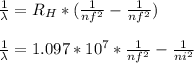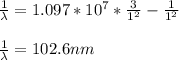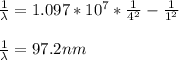Chemistry, 22.09.2021 02:00 Horiizon93
An atomic emission spectrum of hydrogen shows three wavelengths:
121.5 nm,102.6 nm, and 97.23 nm
Assign these wavelengths to transitions in the hydrogen atom.
Drag the appropriate items to their respective bins.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
An atomic emission spectrum of hydrogen shows three wavelengths:
121.5 nm,102.6 nm, and 97.23 nm
Questions



Geography, 25.10.2019 06:43

Mathematics, 25.10.2019 06:43

History, 25.10.2019 06:43



Mathematics, 25.10.2019 06:43



Biology, 25.10.2019 06:43


Mathematics, 25.10.2019 06:43



History, 25.10.2019 06:43

Mathematics, 25.10.2019 06:43

Social Studies, 25.10.2019 06:43

Mathematics, 25.10.2019 06:43

Mathematics, 25.10.2019 06:43