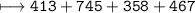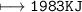Use the reaction and bond information to answer the question.
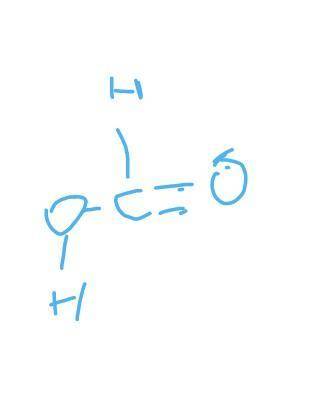
H2 + CO2 → CH2O2
Reactan...

Chemistry, 19.09.2021 14:00 CuteDoggo1828
Use the reaction and bond information to answer the question.
H2 + CO2 → CH2O2
Reactant bond energies: H–H is 432 kJ/mol, C=O is 799 kJ/mol
Product bond energies:
C–H is 413 kJ/mol, C=O is 745 kJ/mol
C–O is 358 kJ/mol, O–H is 467 kJ/mol
How much energy will be given off by the products?
1,570 kJ
1,983 kJ
1,238 kJ
1,516 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2


Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
Questions



History, 21.04.2021 16:00


Physics, 21.04.2021 16:00

Mathematics, 21.04.2021 16:00



Social Studies, 21.04.2021 16:00

Arts, 21.04.2021 16:00

History, 21.04.2021 16:00


Social Studies, 21.04.2021 16:00


Biology, 21.04.2021 16:00



Chemistry, 21.04.2021 16:00

Mathematics, 21.04.2021 16:00