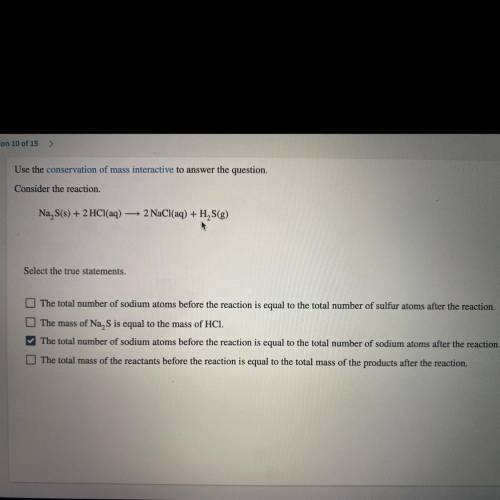
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S...

Chemistry, 19.09.2021 06:20 Emptypockets451
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S(s) + 2 HCl(aq) — 2 NaCl(aq) + H2S(g)
*Select the true statements.*
A. The total number of sodium atoms before the reaction is equal to the total number of sulfur atoms after the reaction.
B. The mass of Na, S is equal to the mass of HCI.
C. The total number of sodium atoms before the reaction is equal to the
total number of sodium atoms after the reaction.
D. The total mass of the reactants before the va reaction is equal to the total mass of the products after the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Questions

Mathematics, 09.03.2021 01:00

Mathematics, 09.03.2021 01:00

English, 09.03.2021 01:00



Mathematics, 09.03.2021 01:00

Mathematics, 09.03.2021 01:00







Biology, 09.03.2021 01:00


Mathematics, 09.03.2021 01:00


Chemistry, 09.03.2021 01:00




