
Chemistry, 19.09.2021 06:10 tasnimsas3
Answer ASAP please
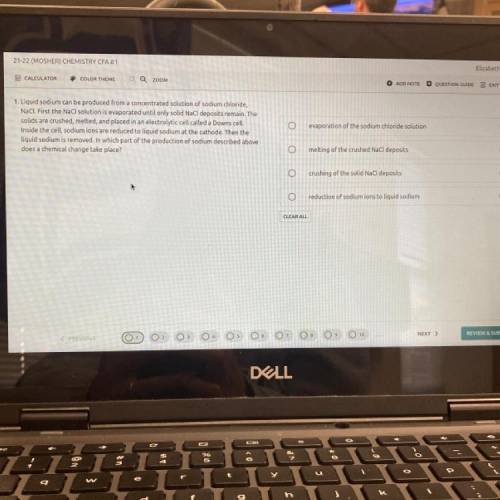
Liquid sodium can be produced from a concentrated solution of sodium chloride,
NaCl. First the NaCl solution is evaporated until only solid NaCl deposits remain. The
solids are crushed, melted, and placed in an electrolytic cell called a Downs cell.
Inside the cell, sodium ions are reduced to liquid sodium at the cathode. Then the
liquid sodium is removed. In which part of the production of sodium described above
does a chemical change take place?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
Answer ASAP please
Liquid sodium can be produced from a concentrated solution of sodium chloride,<...
Questions



Mathematics, 26.01.2020 03:31





Social Studies, 26.01.2020 03:31

Mathematics, 26.01.2020 03:31



Mathematics, 26.01.2020 03:31

Chemistry, 26.01.2020 03:31

Spanish, 26.01.2020 03:31

Mathematics, 26.01.2020 03:31


Mathematics, 26.01.2020 03:31





