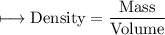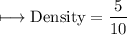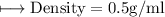Chemistry, 18.09.2021 06:40 rivasalejandro854
A vessel contains 30 mL of water. A sample of 5.0 g of copper metal is dropped into this vessel, raising the level of water in it to 40 mL. What is the density of the copper sample? Please show all steps leading to the final answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
You know the right answer?
A vessel contains 30 mL of water. A sample of 5.0 g of copper metal is dropped into this vessel, rai...
Questions

Mathematics, 04.12.2020 20:50


Mathematics, 04.12.2020 20:50



Mathematics, 04.12.2020 20:50

English, 04.12.2020 20:50

History, 04.12.2020 20:50



Mathematics, 04.12.2020 20:50


Mathematics, 04.12.2020 20:50


Biology, 04.12.2020 20:50



Mathematics, 04.12.2020 20:50

Mathematics, 04.12.2020 20:50