
Chemistry, 18.09.2021 01:00 annemcnair217
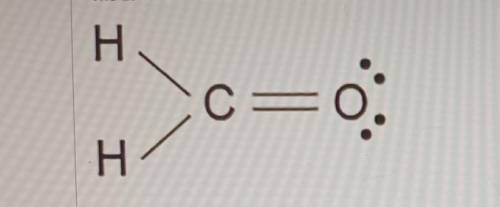
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 points)
O Oxygen is the least electronegative of the three atoms.
O Carbon has a total of four bonded pairs of electrons around it.
O Oxygen has four pairs of non-bonding innermost shell electrons.
O Carbon has an incomplete octet as it transfers an electron to each hydrogen.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 p...
Questions



History, 23.07.2019 13:30

Biology, 23.07.2019 13:30

Social Studies, 23.07.2019 13:30



Computers and Technology, 23.07.2019 13:30







History, 23.07.2019 13:30



History, 23.07.2019 13:30

Biology, 23.07.2019 13:30

History, 23.07.2019 13:30



