
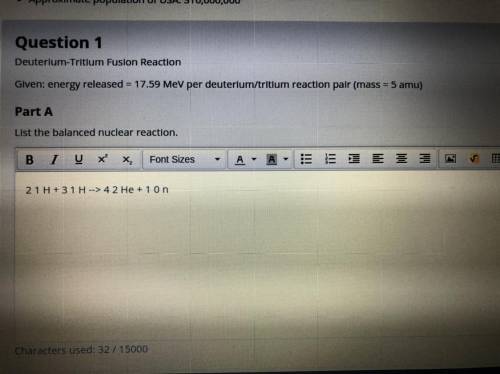
Part A. Deuterium-tritium fusion reaction. Given: energy released = 17.59 MeV per deuterium/tritium reaction pair (mass = 5 amu)
Part B. Determine the energy released per kilogram of fuel used. Given MeV per reaction, calculate energy in joules per kilogram of reactants. Consider 1 mole of tritium plus 1 mole of deuterium yo be a mole of “reactions” (total molar mass = 5 grams)
Part C. Determine the mass of fuel required for the expected energy consumption in the United States for the next 10 years. Energy used per person per year in the United States = 3.5 x 10^11 joules. Base your calculations on a current population of 310,000,000

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1


Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Part A. Deuterium-tritium fusion reaction. Given: energy released = 17.59 MeV per deuterium/tritium...
Questions




Mathematics, 30.11.2020 18:30


English, 30.11.2020 18:30




Biology, 30.11.2020 18:30

Physics, 30.11.2020 18:30

Chemistry, 30.11.2020 18:30


Chemistry, 30.11.2020 18:30

History, 30.11.2020 18:30





History, 30.11.2020 18:30



