
Chemistry, 11.09.2021 01:00 monkey2865
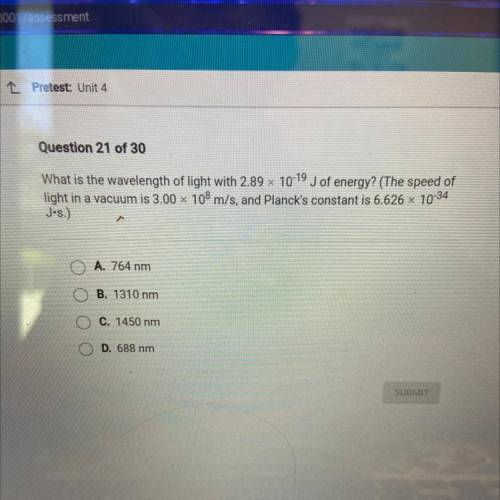
What is the wavelength of light with 2.89 x 10-19 J of energy? (The speed of
light in a vacuum is 3.00 x 108 m/s, and Planck's constant is 6.626 x 10-34
Jos.)
A. 764 nm
B. 1310 nm
C. 1450 nm
D. 688 nm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
What is the wavelength of light with 2.89 x 10-19 J of energy? (The speed of
light in a vacuum is...
Questions


Mathematics, 20.11.2020 07:50

Mathematics, 20.11.2020 07:50



Medicine, 20.11.2020 07:50

English, 20.11.2020 07:50

Mathematics, 20.11.2020 07:50


History, 20.11.2020 07:50

History, 20.11.2020 07:50



Advanced Placement (AP), 20.11.2020 07:50

Mathematics, 20.11.2020 07:50

Mathematics, 20.11.2020 07:50

English, 20.11.2020 07:50



Mathematics, 20.11.2020 07:50



