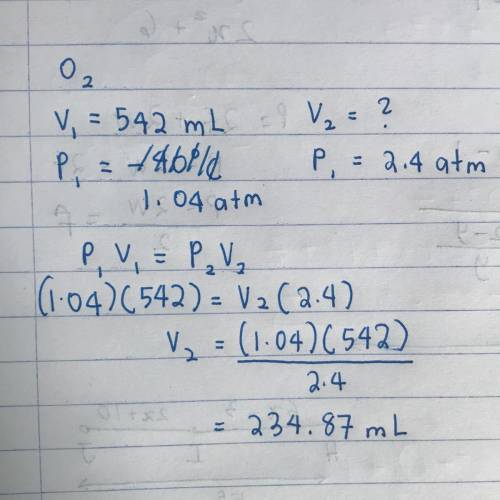When 542 mL of O2 gas at 30°C and 1.04 atm
is cooled to –40 °C and the pressure is in-
creas...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Questions



Mathematics, 17.10.2019 00:30

History, 17.10.2019 00:30



History, 17.10.2019 00:30

Biology, 17.10.2019 00:30


Law, 17.10.2019 00:30

History, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30



Mathematics, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30