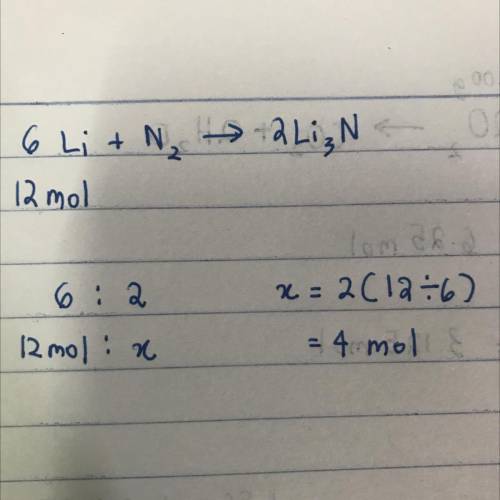Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1

Chemistry, 23.06.2019 13:30
How many ammonium ions and how many sulfate ions are present in a 0.270 mol sample of ?
Answers: 1
You know the right answer?
The equation below shows lithium reacting with nitrogen to produce lithium nitride.6Li + N2 Right ar...
Questions

Social Studies, 27.10.2020 17:50


Spanish, 27.10.2020 17:50

Social Studies, 27.10.2020 17:50

Computers and Technology, 27.10.2020 17:50

Biology, 27.10.2020 17:50




Advanced Placement (AP), 27.10.2020 17:50

Mathematics, 27.10.2020 17:50

Social Studies, 27.10.2020 17:50

English, 27.10.2020 17:50