
Chemistry, 08.09.2021 23:20 jendun123ovrxij
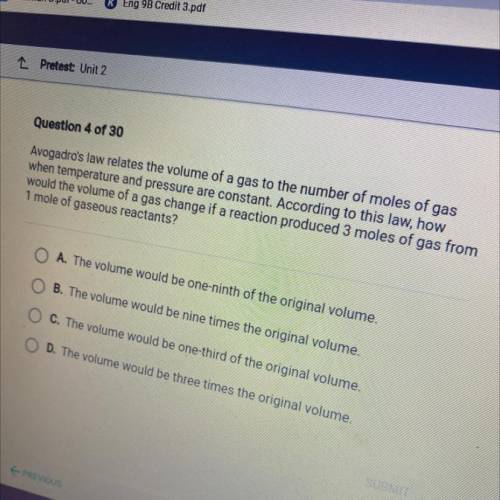
Avogadro's law relates the volume of a gas to the number of moles of gas
when temperature and pressure are constant. According to this law, how
would the volume of a gas change if a reaction produced 3 moles of gas from
1 mole of gaseous reactants?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Avogadro's law relates the volume of a gas to the number of moles of gas
when temperature and pres...
Questions

Mathematics, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50

English, 02.11.2020 23:50




Mathematics, 03.11.2020 01:00

Health, 03.11.2020 01:00

Social Studies, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00



Physics, 03.11.2020 01:00


English, 03.11.2020 01:00

Advanced Placement (AP), 03.11.2020 01:00


Mathematics, 03.11.2020 01:00



