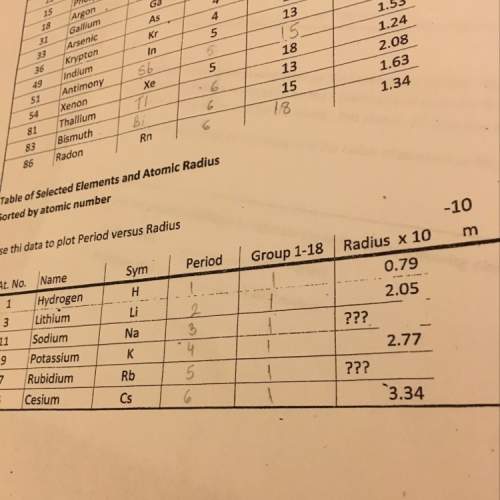
Chemistry, 08.09.2021 02:50 mooneyhope24
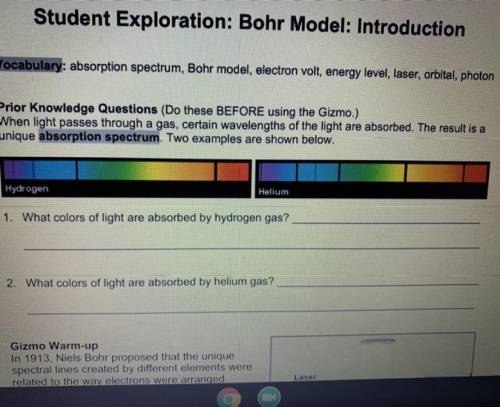
What colors of light are absorbed by hydrogen gas
What colors of light are absorbed by helium gas
Thanks whoever helps :)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 02:30
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
What colors of light are absorbed by hydrogen gas
What colors of light are absorbed by helium gas...
Questions



Computers and Technology, 24.06.2019 12:30

Computers and Technology, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30


Mathematics, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30



History, 24.06.2019 12:30

Computers and Technology, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30

Mathematics, 24.06.2019 12:30


Biology, 24.06.2019 12:30

History, 24.06.2019 12:30