
Chemistry, 07.09.2021 23:40 wwwclarajohnson39
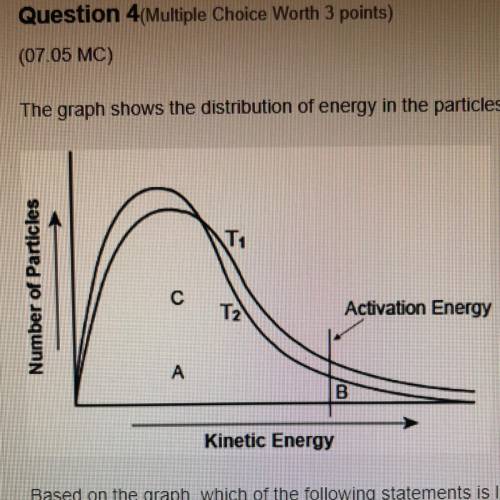
The graph shows the distribution of energy in the particles of two gas samples at different temperatures, T and T2. A, B, and C represent individual particles
Based on the graph, which of the following statements is likely to be true?
Particle A and C are more likely to participate in the reaction than particle B.
Most of the particles of the two gases have very high speeds.
A fewer number of particles of gas at T, are likely to participate in the reaction than the gas at T2
The average speed of gas particles at T2 is lower than the average speed of gas particles at T1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1


Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
The graph shows the distribution of energy in the particles of two gas samples at different temperat...
Questions



Mathematics, 12.04.2021 03:10


Mathematics, 12.04.2021 03:10

Biology, 12.04.2021 03:10


Mathematics, 12.04.2021 03:10


World Languages, 12.04.2021 03:10

Social Studies, 12.04.2021 03:10



Mathematics, 12.04.2021 03:10




Social Studies, 12.04.2021 03:10

Mathematics, 12.04.2021 03:10



