
Chemistry, 07.09.2021 19:40 jpatte2oye8qv
The polyatomic nitrate ion (NO3−) is present in both the products and the reactants of the chemical equation shown below.
Ba(NO3)2 + 2LiOH → Ba(OH)2 + 2LiNO3
How many nitrate ions are in the products and the reactants of the chemical equation?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3


Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
The polyatomic nitrate ion (NO3−) is present in both the products and the reactants of the chemical...
Questions







Computers and Technology, 12.08.2020 22:01





Mathematics, 12.08.2020 22:01






Engineering, 12.08.2020 22:01


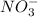 , so we just need to count how many of them are on each side of the equation.
, so we just need to count how many of them are on each side of the equation.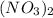 , which shows that there are two nitrate ions in the reactant side (as seen by the little 2).
, which shows that there are two nitrate ions in the reactant side (as seen by the little 2).


