Use the References to access important values if needed for this question.
H
A-Z
The p...

Chemistry, 06.09.2021 05:50 destiny465
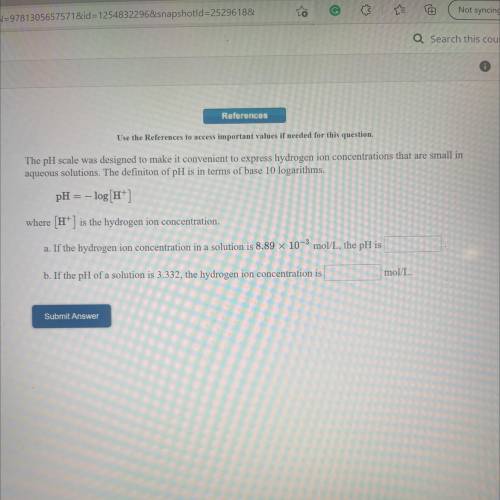
Use the References to access important values if needed for this question.
H
A-Z
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in
aqueous solutions. The definiton of pH is in terms of base 10 logarithms.
pH = -log[H+]
where (H+) is the hydrogen ion concentration.
a. If the hydrogen ion concentration in a solution is 8.89 x 10-3 mol/L, the pH is
b. If the pH of a solution is 3.332, the hydrogen ion concentration is
mol/L.
Submit Answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1
You know the right answer?
Questions

Physics, 27.08.2021 16:30


Biology, 27.08.2021 16:30


English, 27.08.2021 16:30

Mathematics, 27.08.2021 16:30





Chemistry, 27.08.2021 16:40

Mathematics, 27.08.2021 16:40


Mathematics, 27.08.2021 16:40


Chemistry, 27.08.2021 16:40


Social Studies, 27.08.2021 16:40


English, 27.08.2021 16:40



