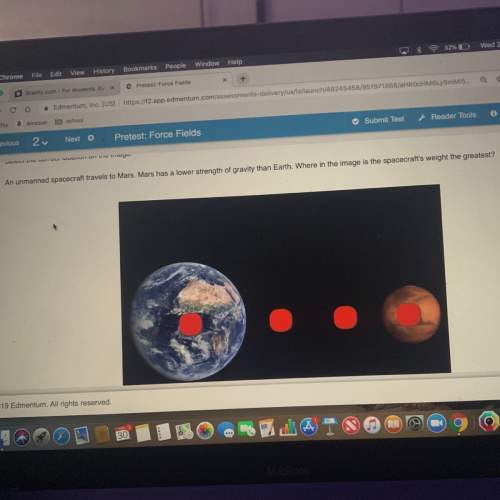
4. Draw the electron dot structures for the following compounds:
a. O3
b. CNCl
c. CH5N...

Chemistry, 03.09.2021 02:10 shayshayyy41
4. Draw the electron dot structures for the following compounds:
a. O3
b. CNCl
c. CH5N
d. XeI4
5. Draw two resonance forms for nitrosyl chloride, ONCl. Which one is better and why?
6. Draw out the expanded version of the molecule with the condensed formula HCCCH2OH. What is the hybridization of each carbon?
7. Use hybridization to explain why carbon can only form four bonds.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Questions


Mathematics, 09.11.2020 18:10

Mathematics, 09.11.2020 18:10

Mathematics, 09.11.2020 18:10



English, 09.11.2020 18:10




English, 09.11.2020 18:10

Mathematics, 09.11.2020 18:10

Arts, 09.11.2020 18:10



English, 09.11.2020 18:10

Social Studies, 09.11.2020 18:10



Mathematics, 09.11.2020 18:10