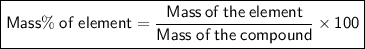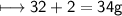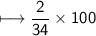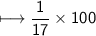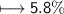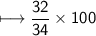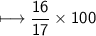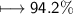Chemistry, 01.09.2021 14:00 ljohnson135
Compound 2 contains 2.0g of hydrogen and 32.0g oxygen. What is the percent compound of each element?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
what is the density of an object that has a mass of 10 g and a volumeof 5 ml? a. 0.5 g/ mlb. 2 g/mlc. 15 g/ mld. 50 g/ ml
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Compound 2 contains 2.0g of hydrogen and 32.0g oxygen. What is the percent compound of each element?...
Questions



Mathematics, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58




Mathematics, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58

Health, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58


Biology, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58



Mathematics, 29.05.2020 08:58

Mathematics, 29.05.2020 08:58