
Chemistry, 30.08.2021 04:20 barstr9146
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histidine at a) pH 5.5 and b) pH 7.0. A solution is provided, but can you do a step-by-step as I do not quite understand the math that went into this.
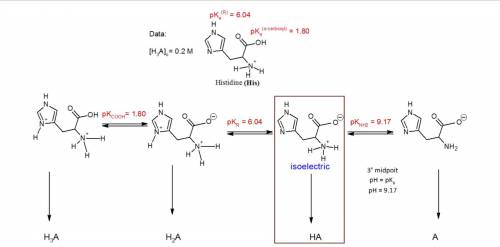
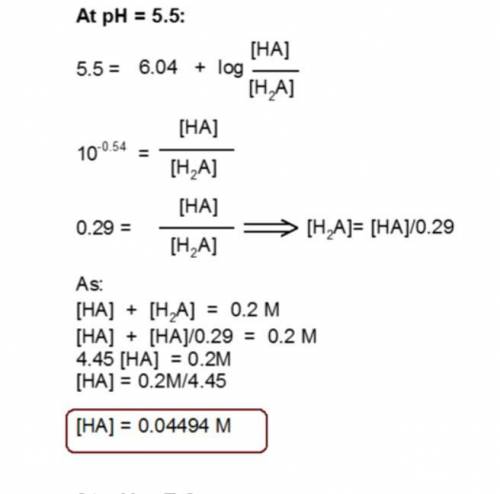

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
You have prepared a .2 M histidine solution. Calculate the molar concentration of isoelectric histid...
Questions




Mathematics, 26.01.2021 14:00


Mathematics, 26.01.2021 14:00



English, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

Physics, 26.01.2021 14:00

English, 26.01.2021 14:00




Mathematics, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

English, 26.01.2021 14:00


Advanced Placement (AP), 26.01.2021 14:00



