
Chemistry, 27.08.2021 08:10 ayoismeisalex
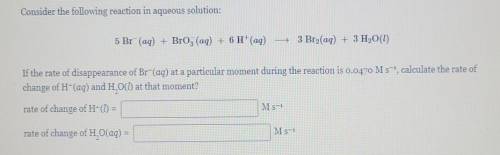
Consider the following reaction in aqueous solution: 5 Br (aq) + Brog (aq) + 6 H+(aq) - 3 Bro(aq) + 3 H2O(l) If the rate of disappearance of Br-(aq) at a particular moment during the reaction is 0.0470 M s-, calculate the rate of change of H-(aq) and H. O() at that moment? rate of change of H- = M s-1 rate of change of H. O(aq) = M s-1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

You know the right answer?
Consider the following reaction in aqueous solution: 5 Br (aq) + Brog (aq) + 6 H+(aq) - 3 Bro(aq) +...
Questions

Mathematics, 08.07.2019 04:30


Chemistry, 08.07.2019 04:30





English, 08.07.2019 04:30

Mathematics, 08.07.2019 04:30

English, 08.07.2019 04:30

Biology, 08.07.2019 04:30

English, 08.07.2019 04:30



Chemistry, 08.07.2019 04:30


Biology, 08.07.2019 04:30

Social Studies, 08.07.2019 04:30


Mathematics, 08.07.2019 04:30



