
Chemistry, 23.08.2021 23:30 ThousandSeas9381
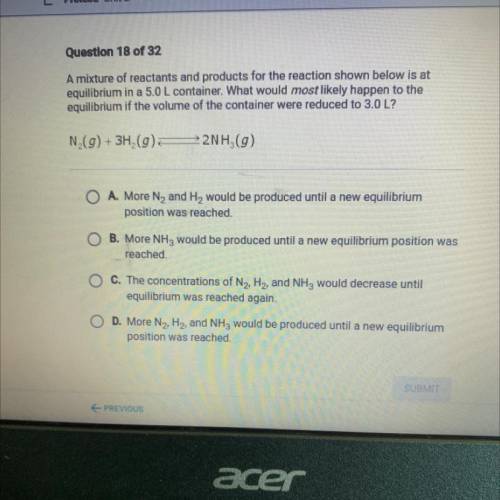
A mixture of reactants and products for the reaction shown below is at
equilibrium in a 5.0 L container. What would most likely happen to the
equilibrium if the volume of the container were reduced to 3.0 L?
N (g) + 3H2(g)
22NH (9)
O A. More N2 and H2 would be produced until a new equilibrium
position was reached.
B. More NH3 would be produced until a new equilibrium position was
reached.
O C. The concentrations of N2, H2, and NH3 would decrease until
equilibrium was reached again.
D. More N2, H2, and NH3 would be produced until a new equilibrium
position was reached.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1


Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 13:30
Type the correct answer in each box. use numerals instead of words. the distance between earth and the moon is about 384,400 kilometers. the distance can be written in scientific notation as a × 10b kilometers, where a = and b = .
Answers: 1
You know the right answer?
A mixture of reactants and products for the reaction shown below is at
equilibrium in a 5.0 L cont...
Questions

Mathematics, 26.02.2020 19:13



Computers and Technology, 26.02.2020 19:13


History, 26.02.2020 19:14






Mathematics, 26.02.2020 19:14








History, 26.02.2020 19:15



