
Chemistry, 23.08.2021 20:10 TheBurntToast
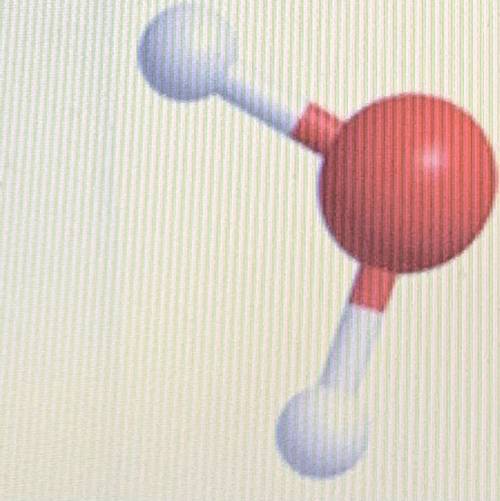
This is a model of a water molecule which has two hydrogen atoms and one oxygen atom. Why ISN'T water considered to be a MIXTURE?
A.
Because the Hydrogen & Oxygen (or O & H) atoms bonded chemically
B.
Because the Hydrogen & Oxygen atoms are easily separated

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
This is a model of a water molecule which has two hydrogen atoms and one oxygen atom. Why ISN'T wate...
Questions











Mathematics, 05.04.2021 23:50


English, 05.04.2021 23:50

Mathematics, 05.04.2021 23:50




Geography, 05.04.2021 23:50




