
Chemistry, 17.08.2021 14:00 milagrosee12
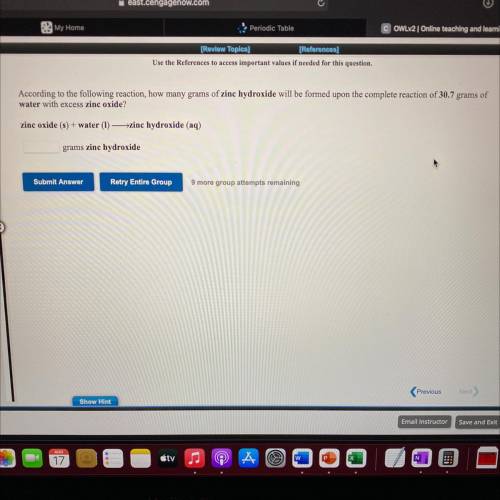
According to the following reaction, how many grams of zine hydroxide will be formed upon the complete reaction of 30.7 grams of
water with excess zinc oxide?
zinc oxide (8) + water (1) -zinc hydroxide (aq)
grams zine hydroxide

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
According to the following reaction, how many grams of zine hydroxide will be formed upon the comple...
Questions

Biology, 23.10.2020 23:30




Biology, 23.10.2020 23:30

Mathematics, 23.10.2020 23:30

Mathematics, 23.10.2020 23:30



Mathematics, 23.10.2020 23:40


Mathematics, 23.10.2020 23:40

Mathematics, 23.10.2020 23:40



Chemistry, 23.10.2020 23:40


Mathematics, 23.10.2020 23:40

Arts, 23.10.2020 23:40



