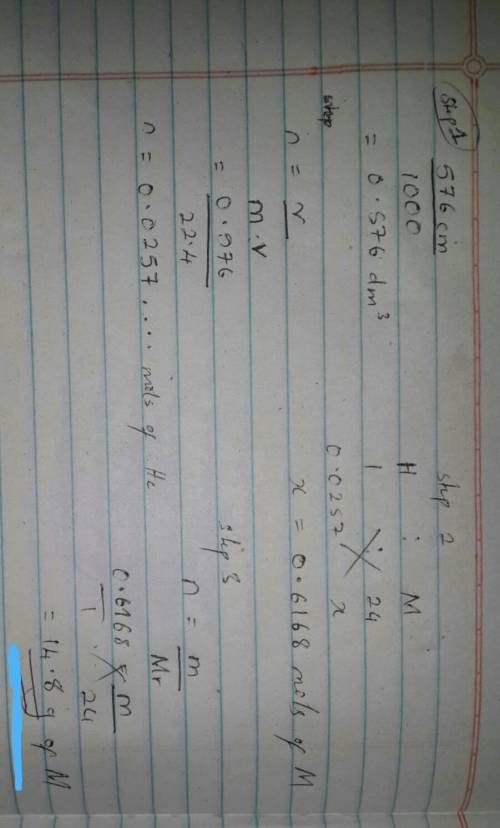Chemistry, 17.08.2021 14:00 laniflower6
An excess of a divalent metal M was dissolved in a limited volume of hydrocloric acid. If 576cm³ of hydrogen was librated at s. t.p , what was the mass of metal that produced this volume of gydrogen?( M = 24, H = 1 , molar volume of gas at s. t.p = 22.4dm³)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
An excess of a divalent metal M was dissolved in a limited volume of hydrocloric acid. If 576cm³ of...
Questions


Mathematics, 27.01.2020 15:31



Mathematics, 27.01.2020 15:31


History, 27.01.2020 15:31

Arts, 27.01.2020 15:31


Chemistry, 27.01.2020 15:31


History, 27.01.2020 15:31

Mathematics, 27.01.2020 15:31



Chemistry, 27.01.2020 15:31