to a solution of H3PO4 (phosphoric acid).

Chemistry, 15.08.2021 03:50 lolomgwtfnvm4
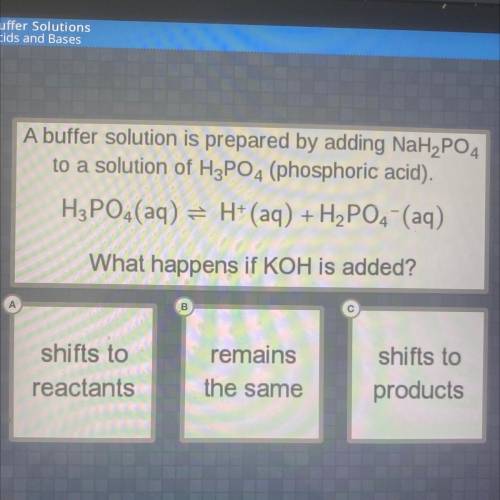
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
H3PO4 (aq) = H+ (aq) + H2PO4 (aq)
What happens if KOH is added?
remains
shifts to
reactants
shifts to
products
the same

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
to a solution of H3PO4 (phosphoric acid).
Questions


Physics, 22.04.2021 20:50



Health, 22.04.2021 20:50

Physics, 22.04.2021 20:50




Mathematics, 22.04.2021 20:50

Mathematics, 22.04.2021 20:50

Mathematics, 22.04.2021 20:50




English, 22.04.2021 20:50



History, 22.04.2021 20:50



