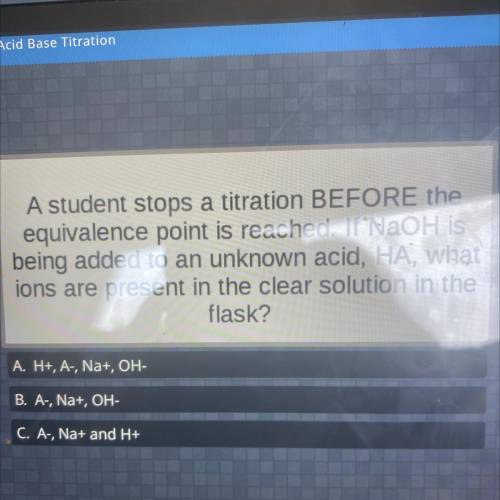
A student stops a titration BEFORE the
equivalence point is reached. If NaOH is
being added t...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Clouds form when water vapor to form small droplets. a. humidifies b. condenses c. evaporates d. precipitates
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Questions

Mathematics, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30

Biology, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30


History, 30.06.2019 17:30







Health, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30

Mathematics, 30.06.2019 17:30

Chemistry, 30.06.2019 17:30

English, 30.06.2019 17:30

Health, 30.06.2019 17:30


Mathematics, 30.06.2019 17:30